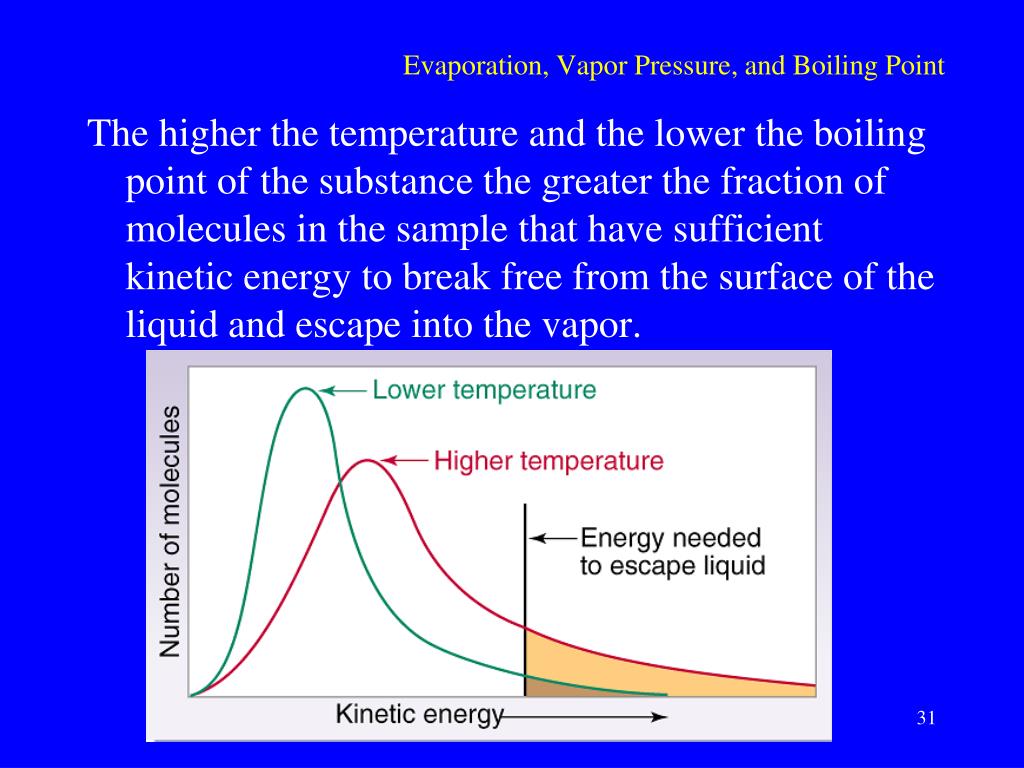
Evaporation Vapor Pressure And Boiling Point . Web the normal boiling point is just the boiling point at atmospheric. Web evaporation, vapor pressure, and boiling point. Web to understand that the equilibrium vapor pressure of a liquid depends on the temperature and the intermolecular forces. Web the boiling point is defined as the temperature at which the saturated vapor pressure of a liquid is equal to the surrounding atmospheric pressure. Web it also looks at how saturated vapor pressure varies with temperature, and the relationship between saturated vapor pressure. Web a liquid’s vapor pressure is directly related to the intermolecular forces. Both vapor pressure and boiling point are affected by the strength of. Web if the partial pressure is less than the vapor pressure, then evaporation will take place, as humidity is less than 100%. Web vapor pressure is the pressure exerted by a vapor above its liquid (or solid) when they are at dynamic equilibrium and in a closed.
from www.slideserve.com
Both vapor pressure and boiling point are affected by the strength of. Web a liquid’s vapor pressure is directly related to the intermolecular forces. Web to understand that the equilibrium vapor pressure of a liquid depends on the temperature and the intermolecular forces. Web vapor pressure is the pressure exerted by a vapor above its liquid (or solid) when they are at dynamic equilibrium and in a closed. Web the normal boiling point is just the boiling point at atmospheric. Web it also looks at how saturated vapor pressure varies with temperature, and the relationship between saturated vapor pressure. Web the boiling point is defined as the temperature at which the saturated vapor pressure of a liquid is equal to the surrounding atmospheric pressure. Web evaporation, vapor pressure, and boiling point. Web if the partial pressure is less than the vapor pressure, then evaporation will take place, as humidity is less than 100%.
PPT Chapter 11 Liquids & Solids PowerPoint Presentation, free
Evaporation Vapor Pressure And Boiling Point Web vapor pressure is the pressure exerted by a vapor above its liquid (or solid) when they are at dynamic equilibrium and in a closed. Both vapor pressure and boiling point are affected by the strength of. Web if the partial pressure is less than the vapor pressure, then evaporation will take place, as humidity is less than 100%. Web the normal boiling point is just the boiling point at atmospheric. Web evaporation, vapor pressure, and boiling point. Web a liquid’s vapor pressure is directly related to the intermolecular forces. Web vapor pressure is the pressure exerted by a vapor above its liquid (or solid) when they are at dynamic equilibrium and in a closed. Web to understand that the equilibrium vapor pressure of a liquid depends on the temperature and the intermolecular forces. Web it also looks at how saturated vapor pressure varies with temperature, and the relationship between saturated vapor pressure. Web the boiling point is defined as the temperature at which the saturated vapor pressure of a liquid is equal to the surrounding atmospheric pressure.
From www.slideserve.com
PPT Phase Changes PowerPoint Presentation, free download ID2437650 Evaporation Vapor Pressure And Boiling Point Web the normal boiling point is just the boiling point at atmospheric. Web the boiling point is defined as the temperature at which the saturated vapor pressure of a liquid is equal to the surrounding atmospheric pressure. Web a liquid’s vapor pressure is directly related to the intermolecular forces. Web if the partial pressure is less than the vapor pressure,. Evaporation Vapor Pressure And Boiling Point.
From www.slideserve.com
PPT Chapter 11 Liquids & Solids PowerPoint Presentation, free Evaporation Vapor Pressure And Boiling Point Web the boiling point is defined as the temperature at which the saturated vapor pressure of a liquid is equal to the surrounding atmospheric pressure. Web vapor pressure is the pressure exerted by a vapor above its liquid (or solid) when they are at dynamic equilibrium and in a closed. Both vapor pressure and boiling point are affected by the. Evaporation Vapor Pressure And Boiling Point.
From www.researchgate.net
The vapor pressure of ethanol vs. the normal boilingpoint temperature Evaporation Vapor Pressure And Boiling Point Web to understand that the equilibrium vapor pressure of a liquid depends on the temperature and the intermolecular forces. Both vapor pressure and boiling point are affected by the strength of. Web it also looks at how saturated vapor pressure varies with temperature, and the relationship between saturated vapor pressure. Web the boiling point is defined as the temperature at. Evaporation Vapor Pressure And Boiling Point.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation, free download ID Evaporation Vapor Pressure And Boiling Point Web a liquid’s vapor pressure is directly related to the intermolecular forces. Web to understand that the equilibrium vapor pressure of a liquid depends on the temperature and the intermolecular forces. Web it also looks at how saturated vapor pressure varies with temperature, and the relationship between saturated vapor pressure. Web vapor pressure is the pressure exerted by a vapor. Evaporation Vapor Pressure And Boiling Point.
From saylordotorg.github.io
Vapor Pressure Evaporation Vapor Pressure And Boiling Point Web if the partial pressure is less than the vapor pressure, then evaporation will take place, as humidity is less than 100%. Web a liquid’s vapor pressure is directly related to the intermolecular forces. Web the boiling point is defined as the temperature at which the saturated vapor pressure of a liquid is equal to the surrounding atmospheric pressure. Web. Evaporation Vapor Pressure And Boiling Point.
From www.numerade.com
From the plot of vapor pressures vs temperature above, estimate the Evaporation Vapor Pressure And Boiling Point Web if the partial pressure is less than the vapor pressure, then evaporation will take place, as humidity is less than 100%. Web a liquid’s vapor pressure is directly related to the intermolecular forces. Both vapor pressure and boiling point are affected by the strength of. Web the boiling point is defined as the temperature at which the saturated vapor. Evaporation Vapor Pressure And Boiling Point.
From www.youtube.com
Intermolecular Forces Trends Melting & Boiling Point, Viscosity Evaporation Vapor Pressure And Boiling Point Web if the partial pressure is less than the vapor pressure, then evaporation will take place, as humidity is less than 100%. Web to understand that the equilibrium vapor pressure of a liquid depends on the temperature and the intermolecular forces. Web the normal boiling point is just the boiling point at atmospheric. Web evaporation, vapor pressure, and boiling point.. Evaporation Vapor Pressure And Boiling Point.
From chem.libretexts.org
Chapter 11.4 Vapor Pressure Chemistry LibreTexts Evaporation Vapor Pressure And Boiling Point Web it also looks at how saturated vapor pressure varies with temperature, and the relationship between saturated vapor pressure. Web the boiling point is defined as the temperature at which the saturated vapor pressure of a liquid is equal to the surrounding atmospheric pressure. Web vapor pressure is the pressure exerted by a vapor above its liquid (or solid) when. Evaporation Vapor Pressure And Boiling Point.
From www.youtube.com
Vapour Pressure and Boiling Point Relationship Vapour Pressure vs Evaporation Vapor Pressure And Boiling Point Web a liquid’s vapor pressure is directly related to the intermolecular forces. Both vapor pressure and boiling point are affected by the strength of. Web the boiling point is defined as the temperature at which the saturated vapor pressure of a liquid is equal to the surrounding atmospheric pressure. Web the normal boiling point is just the boiling point at. Evaporation Vapor Pressure And Boiling Point.
From www.slideserve.com
PPT Chapter 11 Liquids & Solids PowerPoint Presentation, free Evaporation Vapor Pressure And Boiling Point Web a liquid’s vapor pressure is directly related to the intermolecular forces. Web it also looks at how saturated vapor pressure varies with temperature, and the relationship between saturated vapor pressure. Web if the partial pressure is less than the vapor pressure, then evaporation will take place, as humidity is less than 100%. Web the normal boiling point is just. Evaporation Vapor Pressure And Boiling Point.
From sciencenotes.org
Vapor Pressure Definition and How to Calculate It Evaporation Vapor Pressure And Boiling Point Web the boiling point is defined as the temperature at which the saturated vapor pressure of a liquid is equal to the surrounding atmospheric pressure. Both vapor pressure and boiling point are affected by the strength of. Web a liquid’s vapor pressure is directly related to the intermolecular forces. Web vapor pressure is the pressure exerted by a vapor above. Evaporation Vapor Pressure And Boiling Point.
From www.youtube.com
Simplest Way To Understand Boiling Point & Vapor Pressure YouTube Evaporation Vapor Pressure And Boiling Point Web if the partial pressure is less than the vapor pressure, then evaporation will take place, as humidity is less than 100%. Web a liquid’s vapor pressure is directly related to the intermolecular forces. Web vapor pressure is the pressure exerted by a vapor above its liquid (or solid) when they are at dynamic equilibrium and in a closed. Web. Evaporation Vapor Pressure And Boiling Point.
From ppt-online.org
theory of ideal gases презентация онлайн Evaporation Vapor Pressure And Boiling Point Web vapor pressure is the pressure exerted by a vapor above its liquid (or solid) when they are at dynamic equilibrium and in a closed. Web a liquid’s vapor pressure is directly related to the intermolecular forces. Web the normal boiling point is just the boiling point at atmospheric. Web it also looks at how saturated vapor pressure varies with. Evaporation Vapor Pressure And Boiling Point.
From learningdbmuller.z21.web.core.windows.net
Vapor Pressure And Boiling Worksheets Evaporation Vapor Pressure And Boiling Point Web it also looks at how saturated vapor pressure varies with temperature, and the relationship between saturated vapor pressure. Web the boiling point is defined as the temperature at which the saturated vapor pressure of a liquid is equal to the surrounding atmospheric pressure. Web to understand that the equilibrium vapor pressure of a liquid depends on the temperature and. Evaporation Vapor Pressure And Boiling Point.
From www.peoi.org
Chapter 10 Section C Properties of Liquids Evaporation Vapor Pressure And Boiling Point Web it also looks at how saturated vapor pressure varies with temperature, and the relationship between saturated vapor pressure. Web a liquid’s vapor pressure is directly related to the intermolecular forces. Web if the partial pressure is less than the vapor pressure, then evaporation will take place, as humidity is less than 100%. Web to understand that the equilibrium vapor. Evaporation Vapor Pressure And Boiling Point.
From www.youtube.com
Evaporation, Vapor Pressure and Boiling YouTube Evaporation Vapor Pressure And Boiling Point Web a liquid’s vapor pressure is directly related to the intermolecular forces. Web if the partial pressure is less than the vapor pressure, then evaporation will take place, as humidity is less than 100%. Web the boiling point is defined as the temperature at which the saturated vapor pressure of a liquid is equal to the surrounding atmospheric pressure. Web. Evaporation Vapor Pressure And Boiling Point.
From www.slideserve.com
PPT Evaporation, Vapor Pressure, and Intermolecular Forces PowerPoint Evaporation Vapor Pressure And Boiling Point Web the normal boiling point is just the boiling point at atmospheric. Web if the partial pressure is less than the vapor pressure, then evaporation will take place, as humidity is less than 100%. Web a liquid’s vapor pressure is directly related to the intermolecular forces. Both vapor pressure and boiling point are affected by the strength of. Web vapor. Evaporation Vapor Pressure And Boiling Point.
From www.indium.com
Properties of Gallium & Gallium Alloys Indium Corporation Evaporation Vapor Pressure And Boiling Point Web to understand that the equilibrium vapor pressure of a liquid depends on the temperature and the intermolecular forces. Web the boiling point is defined as the temperature at which the saturated vapor pressure of a liquid is equal to the surrounding atmospheric pressure. Web if the partial pressure is less than the vapor pressure, then evaporation will take place,. Evaporation Vapor Pressure And Boiling Point.